February 6, 2023
Personalized drug discovery at lightspeed
HiFiBiO Therapeutics and CSEM are collaborating to develop and manufacture single-cell microfluidics that will enable novel immunotherapies for the treatment of cancer and autoimmune diseases. A new paradigm is emerging thanks to the integration of deep biological expertise and data intelligence.
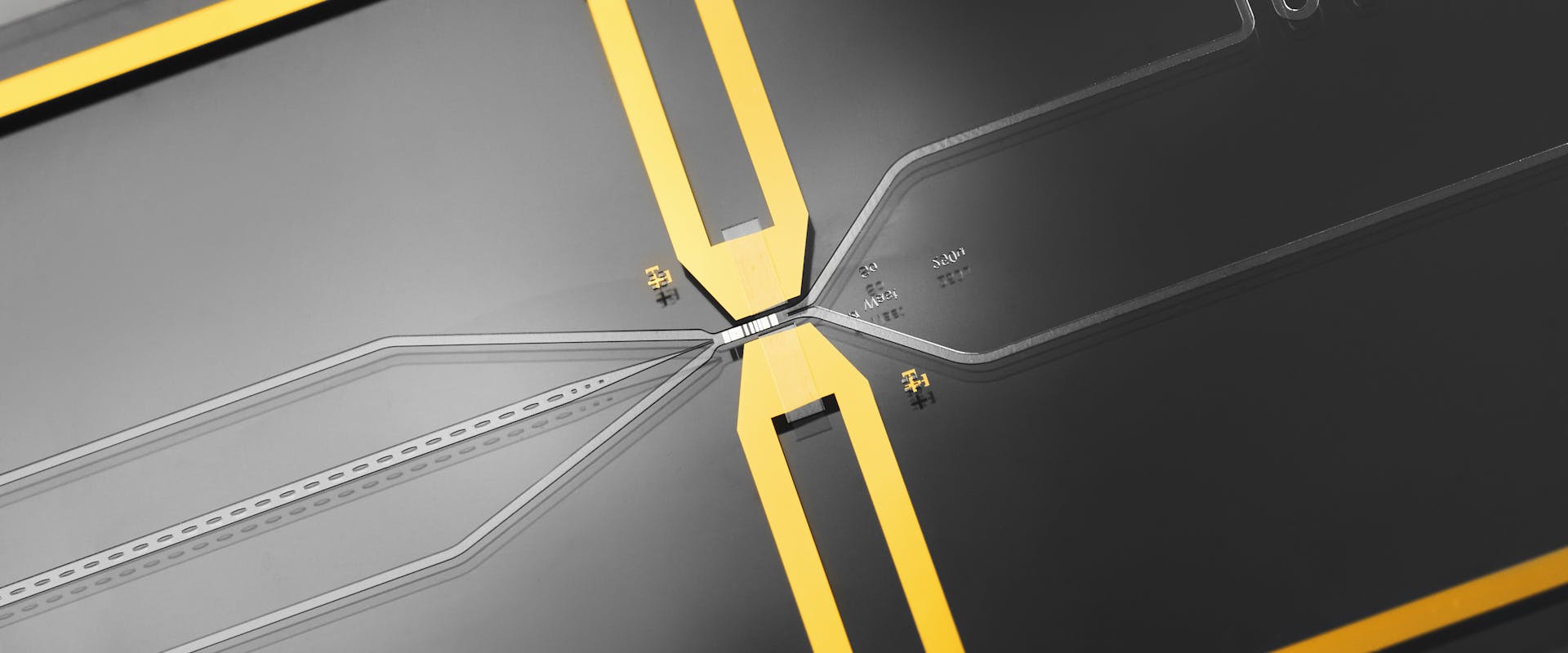
© CSEM - Photograph of the microfluidic chip. The inlets and outlets can be seen, as well as the two gold electrodes that cause the cells to be sorted.


