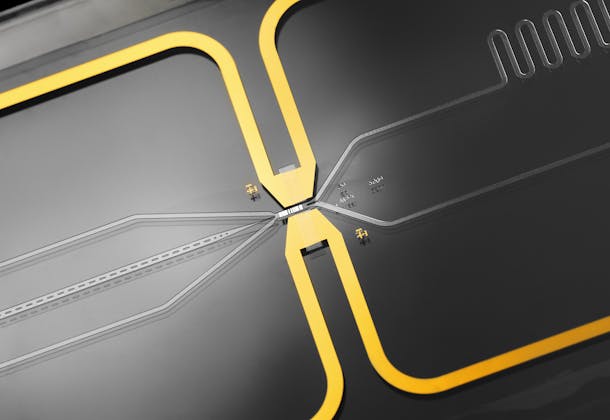
Developing a high performance in-situ filtration membrane
A successful pairing, CSEM’s MEMS team worked with Securecell to develop a specialized and robust microfabrication process that advances certain elements of Sephara; a membrane array that is characterized by its defined pore size and non-random pore distribution. This unique arrangement yields an especially high flow rate and strong capacity to separate particles (cells) of a specific size and shape from a liquid (cell culture broth or blood).
For over a decade now, CSEM have been optimizing their silicon micromachining technologies to “improve flow rates and separation behavior, via specialized techniques that improve pore distribution and density” reports Michele Palmieri, CSEM VP, “we are excited about these methods and the potential they can bring to porous membranes like Sephara.”
Closing the gap and improving bioprocess development
Indeed, Sephara multi-use technology nicely complements Securecell’s existing product portfolio and it boasts some very exciting specifications. These include impressive flow rates and selective filtration with highly reduced fouling and clogging behavior. This is achieved thanks to the thin silicon membrane’s improved pore diameters, which range from 0.2 to 2.2 μm. It also allows the company to address many of the issues associated with particle contamination during downstream purification steps/analytics.
Plus, in certain situations, the probe can also be used as a pre-filtration device to assure early separation of cells from viruses, exosomes, proteins, and metabolites. Carlo Andretta, Securecell's CEO, sees Sephara “become part of modern bioreactors allowing us to capture the full potential of intensified bioprocessing,” thereby closing the gap in real-time cell culture monitoring, bringing analytics closer to the process and improving the understanding of bioprocess development.
Securecell x CSEM
Unveiling the impressive tech and specifications behind Securecell’s Sephara technology also reveals a rewarding collaboration between companies. This is reflected in Carlo’s thoughts, who is thrilled that “after many successful years of Securecell’s product development team working with CSEM’s experts, we have now reached the point where this combined effort of true innovation allows us to offer unparalleled benefits and value to our customers in the biopharma and medical fields.” And he sees these benefits reaching even further in the future, as they will use Sephara “at the heart of SerAccess, [Securecell’s] artificial pancreas project, where glucose measurement and insulin delivery directly happens in the bloodstream,” with the hope that one day they can radically change the treatment of diabetes.